External Events
Online
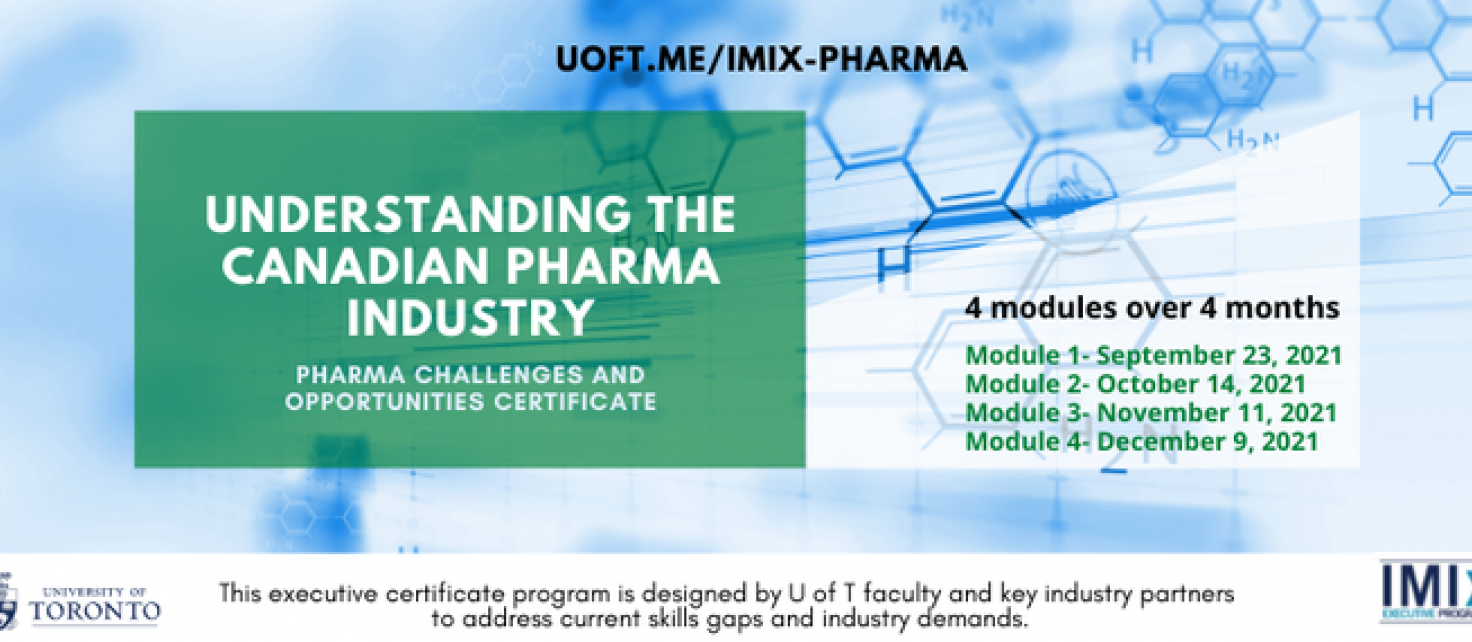
The Pharma Challenges and Opportunities Certificate delivers industry knowledge and soft skills that pharma CEOs identified as important. This program will consist of four 1/2 day modules delivered by experts from industry as well as distinguished U of T faculty. It is designed to be highly interactive including workshops, case studies, role-play and networking opportunities.
This program will provide a deep understanding of the journey of a medicine from discovery through regulatory approval, access to the Canadian market and activities related to launching, marketing and selling. It will also cover the broader Canadian life sciences such as generics, vaccines and key future opportunities and challenges.
Online Modules and Learning Outcomes
Module 1
Introduction to the industry • R&D • Regulatory Affairs • Manufacturing and Quality
Introduction to the industry, discovering, developing, and manufacturing a new medicine and navigating the regulatory process to gain approval to market.
Module 2
Canadian Drug Healthcare System • Market Access • Public Policy
Overview of Canadian drug policy framework and processes for gaining market access for a new medicine. Understanding the stakeholders and their roles in public and private markets.
Module 3
Launching • Marketing and Selling Best Practice • Persuasion and Influence
Introduction to best practices in launching, marketing and selling pharmaceutical products in Canada.
Module 4
Vaccines • Generics • Biologics and Life Sciences in Canada
A look at the broader life sciences ecosystem in Canada and future opportunities and challenges.
Certificate Fee
U of T Alumni rate: $1650.
Current U of T PhD students can register for $650+HST.